1. INTRODUCTION
In septic pulmonary embolism (SPE), a relatively rare form of pulmonary embolism, the thrombus, which contains pathogens, is mobilized from the site of infection and implanted into the pulmonary vascular system [1, 2]. Staphylococcus aureus is the most common pathogen [3]. In a case series analyzing 60 patients with SPE, 78% were intravenous drug users [4]. Staphylococcus aureus causes SPE, and is associated with a high mortality rate and poor prognosis. In addition to comprehensive clinical assessment and laboratory examination, radiological examination plays a key role in the diagnosis and management of SPE. This article describes a patient with newly diagnosed diabetes whose initial symptom was SPE associated with Staphylococcus aureus pneumonia, combined with pulmonary tuberculosis and bronchial tuberculosis. Our aim was to improve clinicians’ awareness of early diagnosis and treatment for this type of patient and improve patient prognosis.
2. CASE PRESENTATION
2.1 General information
A 61-year-old woman was admitted to the hospital on March 24, 2023. Three days earlier, she had a noticeable fever. Because of hemoptysis, she underwent a chest CT scan at another hospital on March 23, which showed multifocal strip-like and nodular shadows in both lungs. She felt chest tightness and discomfort after physical activity, and received a consultation at the respiratory department of our hospital on March 24. At admission, her temperature was 38.2°C, her blood pressure was 139/70 mmHg, her respiratory rate was 25 breaths/minute, her pulse was 130 beats/minute, and her SPO2 concentration was 92% (without oxygen). She was immediately given nasal cannula oxygen therapy because of difficulty breathing and chest pain after admission. An urgent enhanced chest CT scan revealed multifocal large consolidated shadows in both lungs. The lesions in both lower lungs were significantly greater than they had been on the previous day, but no significant pulmonary vascular embolism was observed ( Figure 1 ). Enhanced CT ( Figure 2 ), blood culture, blood mNGS, routine blood tests, coagulation function tests, and blood gas analysis were immediately performed.

The patient underwent a chest CT scan because of hemoptysis.
The lesions in the upper lobes of both lungs were pulmonary tuberculosis (a1, a2, d1, d2, g1, g2). On the second day, her chest tightness was severe, and a CT scan (d1, d2, c1, c2, f1, f2) was performed. A large flake solid shadow was observed in the lower lobes of both lungs (which appeared rapidly within 1 day). CT re-examination 1 month after discharge (g1, g2, h1, h2, j1, j2) showed that the lesions in the lower lobes of both lungs were essentially absorbed.
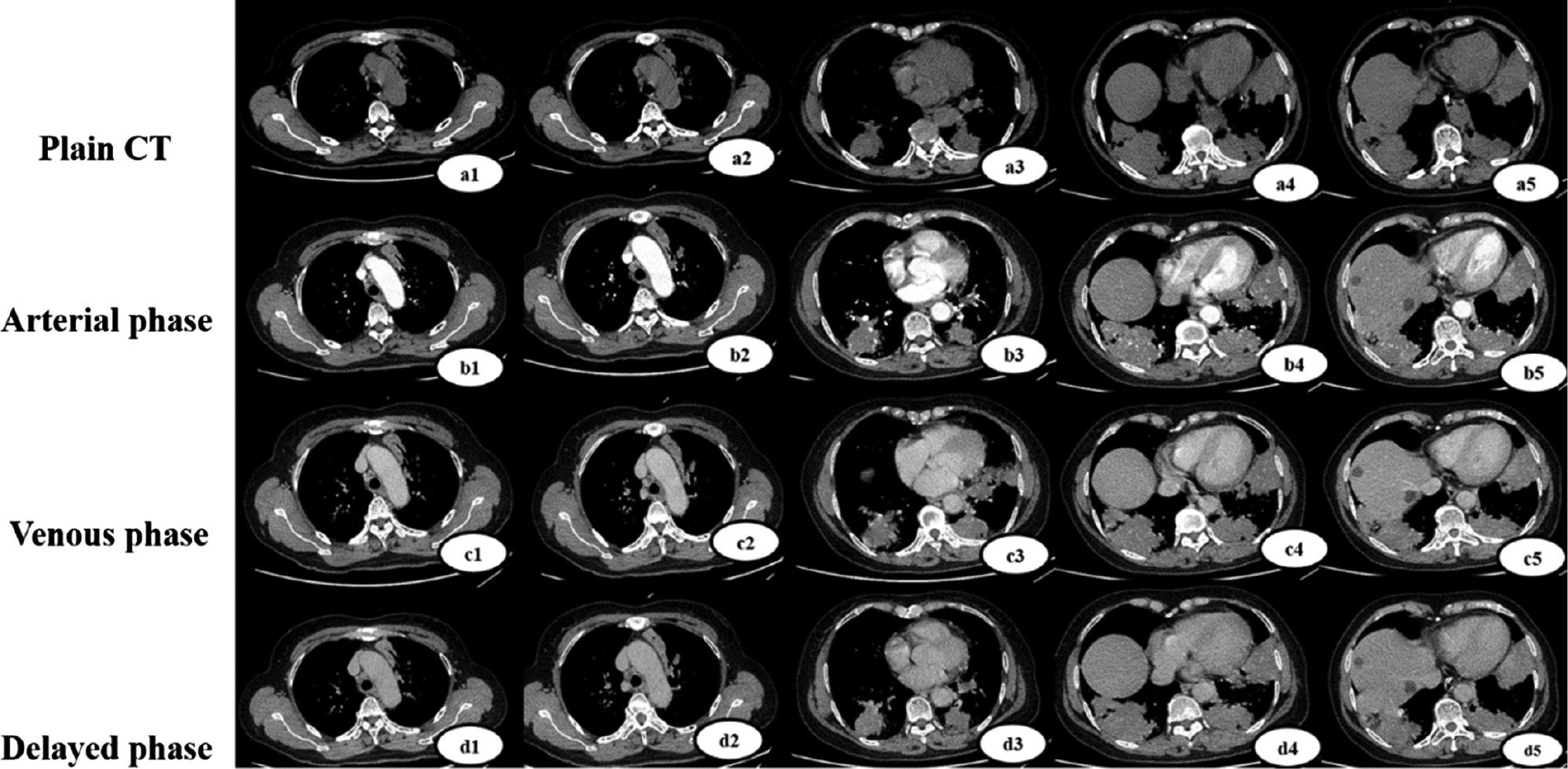
The patient underwent an emergency enhanced CT scan.
Plain-scan, arterial-phase, portal-phase, and delayed-phase pulmonary tuberculosis lesion enhancement (a1, b1, c1, d1, a2, b2, c2, d2). Enhancement of SPE lesions in the plain, arterial, portal, and delayed phases (a3-a5, b3-b5, c3-c5, d3-d5).
2.2 Examination
The results of blood gas analysis, complete blood analysis, hypersensitive C-reactive protein (CRP) level, procalcitonin (PCT) level, coagulation indices, cardiac marker levels, and erythrocyte sedimentation rate on March 24, 2023, and at re-examination after 1 week, are shown in Table 1 . Peripheral blood mNGS revealed Staphylococcus aureus, with 196 reads, and Haemophilus influenzae, with 232 reads, on March 24, 2023. The blood culture (March 24, 2023) was negative, but a blood interferon gamma release assay was positive. The sputum X-pert result was weakly positive. The serum cryptococcal antigen test, serum antineutrophil cytoplasmic antibodies, Aspergillus IgE, and Aspergillus IgG antibody test results were all negative. Sputum fungal immunofluorescence, sputum total bilirubin, and sputum total bilirubin (TB-DNA) were negative. Bedside bronchoscopy was completed with the assistance of high-flow respiratory fluid on March 30, 2023. The tracheal mucosa was congested and swollen, a large amount of purulent sputum was visible, and granulation tissue and caseous-like matter attached on the surface were visible on the carina of the trachea. Granulation tissue and caseous-like matter were visible on the surface of the left main bronchus; the mucosa of the upper and lower lobe bronchus on the left side was clearly congested; a large amount of purulent sputum was visible in the right bronchus; and a large amount of caseous-like matter and purulent sputum obstructed the lumen in the right upper lobe. After suction, the opening of the right upper lobe was clearly narrowed; moreover, the mucosa was congested, swollen, and eroded, and easily bled when touched. Granulation was observed on the wall of the right lower lobe, and a small amount of caseous-like matter and granulation were observed at the base of the right lower lobe. The diagnosis was bronchial tuberculosis ( Figure 3 ). Bronchoalveolar lavage fluid (BALF) and smears for acid-fast bacilli, BALF Xpert, BALF TB-DNA, and BALF-GM were negative. BALF general bacterial culture revealed Staphylococcus aureus 2+. BALF-mNGS revealed Staphylococcus aureus species, with 9734 reads; Haemophilus influenzae species, with 12 reads; and Mycobacterium tuberculosis complex species, with one read. No atypical cells were observed in the bronchoscopic BALF or brush smear pathological examination.

a. Granulation tissue and caseous-like matter attached to the carina of the trachea. b. Granulation tissue was observed in the bronchial wall of the lower lobe of the right lung. c. Patient material and granulation tissue were found in the bronchus of the basal segment of the lower lobe of the right lung. d. A caseous material attached to the granulation surface was observed on the left main bronchial wall. e. The bronchial mucosa of the lower lobe of the left lung was congested.
Comparison of relevant laboratory indices on March 24, 2023, and after 1 week.
| Test item | Index | March 24, 2023 | Re-examination after 1 week |
|---|---|---|---|
| Blood gas analysis | pH | 7.467 | 7.451 |
| PCO2 | 31.0 mmHg | 37.6 mmHg | |
| PO2 | 61.9 mmHg | 143.2 mmHg | |
| HCO3− | 21.9 mmol/L | 28.7 mmol/L | |
| Lactate | 1.4 mmol/L | 2.0 mmol/L | |
| Oxygenation index | 137.45 mmHg | 286.47 mmHg | |
| Erythrocyte sedimentation rate | 47 mm/h | - | |
| Complete blood count | White blood cell count | 4.48×109/L | 6.01×109/L |
| Neutrophil percentage | 85.30% | 83.80% | |
| Platelet count | 141×109/L | 297×109/L | |
| Lymphocyte percentage | 8.70% | 10.00% | |
| High-sensitivity CRP | 160 mg/L | 32.6 mg/L | |
| PCT | 36.090 ng/ml | <0.1 ng/mL | |
| Coagulation indicators | D-dimer | 3100 μg/L | Normal |
| Fibrinogen | 5.09 g/L | Normal | |
| Cardiac markers | NT-proBNP | 506 pg/mL | Normal |
| Troponin | 13 pg/mL | Normal | |
| Myoglobin | 133.6 ng/ml | Normal | |
| Tumor markers | Serum CEA | Normal | Normal |
| Biochemistry | Hemoglobin A1c | 7.30% | - |
| Albumin | 40.5 g/L | 28.0 g/L |
2.3 Diagnosis
(A) Septic pulmonary embolism. (B) Staphylococcus aureus-induced pneumonia. (C) Multidrug-resistant pulmonary tuberculosis (isoniazid and ethambutol drug-resistant). (D) Bronchial tuberculosis. (E) Hemoptysis. (F) Type I respiratory failure. (G) Electrolyte disorder. (H) Type 2 diabetes. (I) Malnutrition.
2.4 Treatment
High-flow oxygen therapy was administered to strengthen respiratory support, along with empirical anti-infective treatment with cefotaxime sodium and moxifloxacin. Hemostasis was achieved, nutritional support was strengthened, water and electrolyte balance were maintained, blood pressure was monitored, fluid input and output were balanced, and blood sugar was actively controlled. Later, on the basis of blood mNGS indicating Staphylococcus aureus, cefotaxime sodium was discontinued, and the antibiotic meropenem was upgraded and combined with linezolid for anti-infective treatment. After 1 week of treatment, the patient’s PCT level returned to the normal range, her body temperature returned to normal, her respiratory symptoms significantly improved, and a recheck CT of the chest showed a partial decrease in multiple solid changes in the lower lobes of both lungs. After the patient’s condition improved, bedside bronchoscopy was performed, and pulmonary tuberculosis and bronchial tuberculosis were diagnosed. Isoniazid, rifampicin, ethambutol, and pyrazinamide were used in combination with isoniazid inhalation therapy for bronchial tuberculosis. After approximately two additional weeks of treatment, the patient’s condition stabilized and improved, and she was discharged from the hospital.
2.5 Treatment results, follow-up, and outcomes
The patient’s BALF was cultured for Mycobacterium tuberculosis and drug sensitivity (HE resistant). After 6 weeks of treatment with linezolid, a repeated chest CT ( Figure 1 . C1-C3, c1-c3) indicated possible tuberculosis lesions in both upper lungs. Multiple solid changes in both lower lungs had been substantially absorbed, in contrast to the prior findings. Eventually, the treatment was adjusted to rifampicin, pyrazinamide, and moxifloxacin for anti-tuberculosis treatment. Her blood sugar level was stable.
3. DISCUSSION
This case involved a rare clinical instance of a newly diagnosed older female patient with diabetes and Staphylococcus aureus infection leading to SPE, who concurrently had multidrug-resistant tuberculosis and bronchial tuberculosis. The patient’s initial clinical symptoms were acute onset, fever, difficulty breathing, chest pain, and hemoptysis, with abnormally elevated infection indicators (PCT: 36.09 ng/mL) and rapidly progressing large consolidation shadows in both lower lungs, accompanied by rapid cavitation and hypoxemia (SQFA=3; qSOFA=1, NEWS=8), thus leading to the diagnosis of SPE.
Because of the patient’s elevated D-dimer levels, an enhanced CT scan was immediately completed after admission to rule out vascular pulmonary embolism. A chest CT scan showed a soft tissue density shadow in the bilateral subpleura in the basal segment of the lower lobes of the lungs, with several cuneiform changes (rapid emergence), unclear boundaries, and halo signs consistent with sepsis-related pulmonary embolism. On an enhanced CT scan, no clear signs of pulmonary embolism were found, and emboli were extremely rare. This aspect is key to distinguishing SPE from other types of pulmonary embolism. The feeding vessel sign [5, 6] has been reported in the literature as a characteristic of SPE. In our patient, we performed emergency CT imaging within 24 hours of acute onset, and feeding vessel signs were found. During the course of disease progression, a cavity forms in approximately 50% of patients [3]. On CT, the patient’s subpleural lesions in the lower lobes of both lungs rapidly formed a cavity. Because of the patient’s critical condition and extreme difficulty breathing at that time, many motion artifacts were present in the CT images, but voids were still observed because of infection. Some lung tissue lesions may become necrotic and form cavities containing gas and fluid. These radiographic characteristics typically require a differential diagnosis among conditions such as pulmonary embolism, lung abscess, and pulmonary metastasis. Chest CT findings may be the first indication of the diagnosis, which serves as the visual basis for early diagnosis of SPE. In this case, combined with the clinical history and laboratory data, SPE was considered to be caused by S. aureus infection.
Given the patient’s acute bloodstream infection, immediate blood culture and blood mNGS were performed, and empirical antimicrobial therapy was initiated. After blood mNGS confirmed S. aureus infection, the antimicrobial spectrum was immediately adjusted. Respiratory support was strengthened, and the water and electrolyte balance and homeostasis were maintained; subsequently, the patient’s infection was effectively controlled after 1 week. This patient had newly diagnosed diabetes with poor blood sugar control, which may be a key factor causing S. aureus-related SPE.
SPE is a rare type of pulmonary embolism [7] involving obstruction of the pulmonary artery by a clot containing pathogens, thereby leading to pulmonary arteriole embolism (or pulmonary lobule infarction), septicemia, and focal pulmonary abscess. The pathophysiological basis of SPE involves multiple processes, including infection, thrombosis, embolic events, inflammatory reactions, and tissue damage. The infection spreads from the pulmonary artery wall to the periphery, where it can form nodules or patchy infiltrates in the lungs [8]. Because SPE results in the formation of pus in the blood of patients with sepsis and is not caused by blood clotting disorders, the use of anticoagulant drugs has no effect. The primary manifestations are septicemia, respiratory symptoms (chest pain, cough, and hemoptysis), and focal lung infiltration [9]. Chronic kidney disease and diabetes are the most common comorbidities [10]. CT pulmonary angiography [11] is often the preferred imaging method for determining clinical respiratory distress. Because the treatment of nonthrombotic pulmonary embolism is completely different from that of thrombotic pulmonary embolism, distinguishing between these conditions is crucial [12]. The most significant CT feature of SPEs is multiple peripheral nodules and peripheral wedge-shaped shadows in both lungs, with or without cavitation. Early recognition and treatment are crucial in the management of SPE. If diagnosis is delayed, thus resulting in untimely treatment, the prognosis is poor. Although SPE is usually associated with bacteria such as Staphylococcus aureus [3, 9, 13, 14], infections with Klebsiella pneumonia [15], Streptococcus mutans [16], and fungi [3] have also been observed to cause SPE. A retrospective study has revealed that patients with gram-positive septicemia are more likely to have cavitated nodules and signs of bronchial inflation than patients with gram-negative bacteremia. The CT features of patients in the gram-negative group were a “halo sign,” “feeding vessel sign,” and clear nodules [17].
The high mortality rate of SPE is due primarily to concurrent septic shock and multiple-organ failure. In recent years, the prognosis of SPE has significantly improved, owing to early diagnosis, more effective antibiotic treatment, early aggressive surgical intervention, and better supportive care [18]. The correct and rational use of antimicrobial drugs is key in successful sepsis treatment. The patient was immediately admitted for blood culture and blood mNGS testing. The blood culture was negative, and blood mNGS was performed only after 1 day, thus providing a reliable basis for the selection of effective antibiotics for Staphylococcus aureus in our patient. The advantage of second-generation sequencing detection in infectious disease diagnosis [19] is that it can detect pathogens that other traditional methods cannot detect, and has a clear advantage in time effectiveness, thus improving the ability to detect pathogens in central nervous system infections, bloodstream infections, respiratory infections, and other localized infections. mNGS play an important role in the early diagnosis of SPE. Its advantages include independent of culture, rapid speed, high sensitivity, and little interference with antibiotic treatment. Compared with blood culture, mNGS offers rapid pathogen detection and aligns more closely with the clinical diagnosis of sepsis. However, the interpretation of mNGS results should include clinical symptoms, laboratory-related inflammation indices, and blood culture results, to improve the reliability of the clinical diagnosis of sepsis [20, 21].
The patient’s acute Staphylococcus aureus infection was effectively controlled. Based on the weakly positive result of the Xpert test on the patient’s sputum, the positive result of the blood interferon gamma release assay (IGRA) test, and the characteristics of the lesions in both upper lungs consistent with tuberculosis, the diagnosis of concurrent pulmonary tuberculosis was made. The patient was an older woman newly diagnosed with diabetes, who had poor blood sugar control and was prone to multiple infections by agents such as tuberculosis, bacteria, and fungi. However, after effective anti-infection treatment, the patient stopped producing phlegm, and a sputum sample could not be obtained for further tuberculosis pathogen testing. Therefore, under the support of a high-flow respiratory aid, bedside bronchoscopy was performed, and bronchial tuberculosis was assessed microscopically. BALF-mNGS revealed Staphylococcus aureus and tuberculosis. Conventional bacterial culture of BALF indicated Staphylococcus aureus, and a drug sensitivity test indicated that the cells were sensitive to linezolid and moxifloxacin. On the basis of further test results, treatment with linezolid and moxifloxacin was continued, and HRZE was added in combination with isoniazid inhalation therapy for bronchial tuberculosis. After more than 1 month of combined anti-infection and anti-tuberculosis treatment, a follow-up chest CT scan indicated slight absorption of the lesions in both upper lungs, and the large consolidated lesions in both lower lungs were essentially absorbed. Subsequent BALF tuberculosis culture indicated the presence of Mycobacterium tuberculosis (HE drug-resistant). On the basis of the results of the drug sensitivity test, the therapy was adjusted to rifampicin, pyrazinamide, and levofloxacin to continue anti-tuberculosis drug treatment. These proactive measures ultimately enabled us to shift from empirical treatment to targeted treatment. In this case report, we focused on the presentation and characteristic CT imaging features of SPE and the application value of mNGS. This case study demonstrated the value of imaging examination combined with mNGS for the timely diagnosis and management of this disease in a population with severe pneumonia complicated by diabetes.
