Abbreviations: PCI, percutaneous coronary intervention; MACEs, major adverse cardiac events; MLD, minimum lumen diameter; QCA, quantitative coronary angiography; OCT, optical coherence tomography; TLR, target lesion revascularization; DAPT, dual antiplatelet therapy; HBR, high bleeding risk.
Introduction
Percutaneous coronary intervention (PCI) with implantation of drug-eluting stents is an effective and safe method for revascularization in patients with coronary heart disease [1]. Drug-eluting stents, compared with bare metal stents, result in lower incidence of in-stent restenosis and target vessel revascularization [2, 3]. Differences in stents’ strut thickness, base polymers, and drug elution also affect device- and clinical-oriented outcomes [4, 5]. Although the advent of second-generation drug-eluting stents decreased the incidence of late stent thrombosis, restenosis and stent thrombosis remain complex challenges. Two major causes of stent failure in the “late catch-up” phenomenon are ischemia-driven target lesion revascularization (TLR) and late restenosis occurring beyond 1 year [6, 7].
The XIENCE everolimus-eluting stent (Abbott Vascular, Santa Clara, CA, USA), a classical second-generation stent, has achieved superior outcomes as well as efficacy compared with first-generation paclitaxel- and sirolimus-eluting stents in patients undergoing PCI, and shows no late catch-up phenomenon at 3 or 5 years [8–10]. The BuMA Supreme sirolimus-eluting stent (SINOMED, Tianjin, China) consists of a stainless-steel thin strut (80 μm) and thin (200 nm) electrografting base, which is top-coated with a biodegradable coating that releases sirolimus relatively quickly, within 28 days, and has shown faster strut coverage than the XIENCE at 1 and 2 month follow-ups [11].
Some trials comparing the clinical outcomes of biodegradable polymers versus second-generation durable polymer drug-eluting stents have indicated similar safety and efficacy profiles [12, 13]. The CENTURY II trial, an intracoronary imaging study, has shown no significant differences in mean neointimal thickness between bioresorbable polymer sirolimus-eluting stents and XIENCE stents at a 9 month optical coherence tomography (OCT) follow-up [14], however, there is a lack of data regarding long-term outcomes.
To date, high-resolution OCT has been the modality most widely used to identify neointimal hyperplasia. LLL is considered a reliable surrogate and valid discriminator for distinguishing stent performance [15]. The aim of the present study was to identify LLL, neointimal tissue coverage, and the “late catch-up” phenomenon of BuMA Supreme stents compared with XIENCE stents implanted in patients with high bleeding risk (HBR), through serial OCT follow-up at within 2 months and 2 years. Patients with HBR were included in the current trial. To our knowledge, no data regarding the relationship between bleeding propensity and neointimal proliferation after stenting are available in the public domain.
Methods
Study Design and Patients
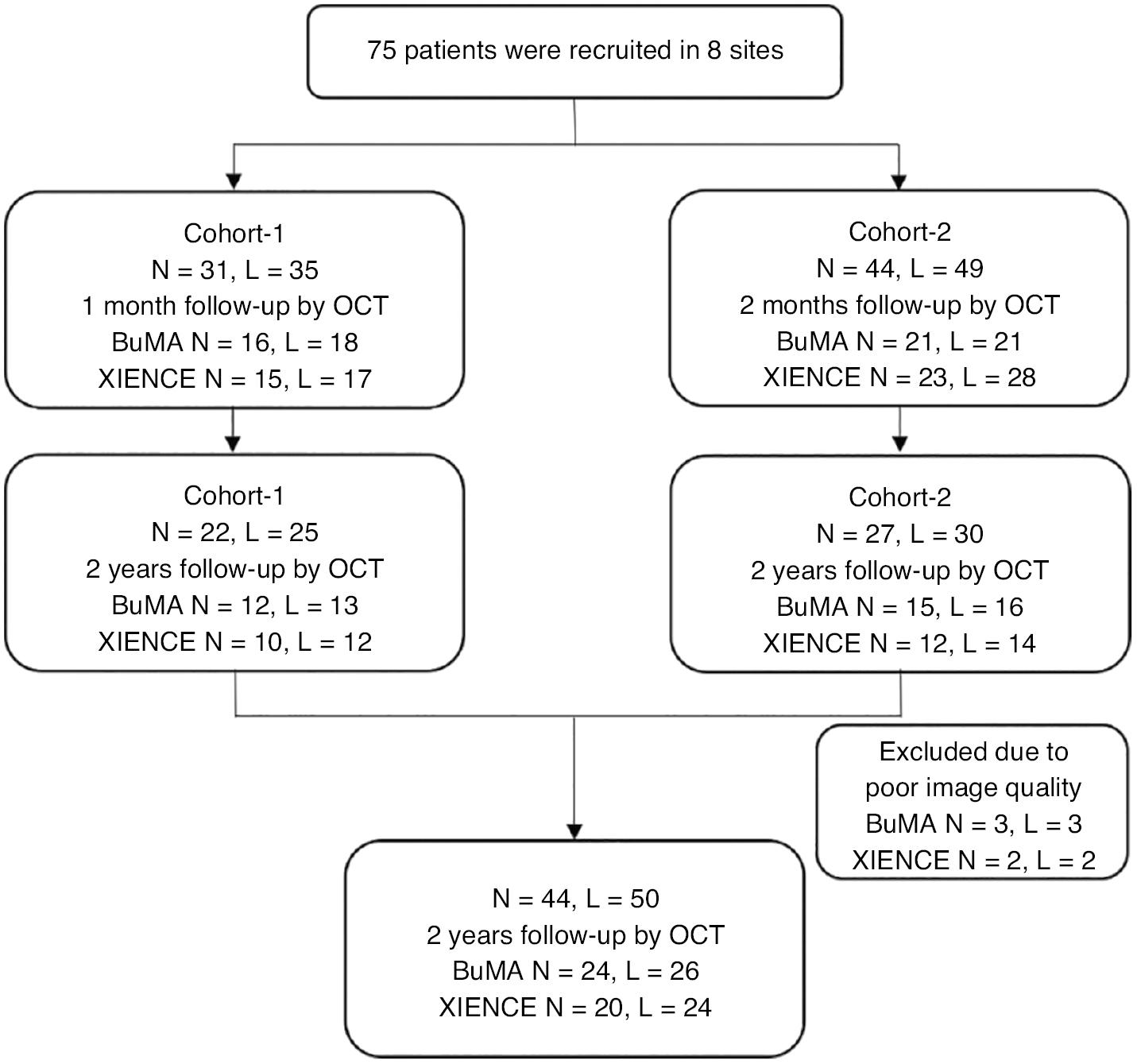
A total of 75 patients with coronary heart disease referred for PCI were enrolled in the PIONEER-II OCT trial; 31 patients were assigned to cohort 1 (BuMA: n = 16, XIENCE: n = 15), and 44 patients were assigned to cohort 2 (BuMA: n = 21, XIENCE: n = 23). A total of 26 patients declined to continue OCT follow-up, and 49 patients from cohort 1 and cohort 2 completed the final 2 years of OCT follow-up. Five patients (three in the BuMA Supreme arm and two in the XIENCE arm) were excluded because of poor image quality. Consequently, 44 patients were included in the statistical analysis: 24 patients with 26 lesions in the BuMA Supreme group and 20 patients with 24 lesions in the XIENCE group (Figure 1). The inclusion and exclusion criteria and the central randomized method based on a computer system were as previously described [11, 16].
The present study was conducted in accordance with the Declaration of Helsinki. The PLA General Hospital Ethics Committee Board approved the protocol (approval number: 2016-018), and all patients provided written informed consent to participate.
Coronary Angiography
A loading dose of aspirin (300 mg) and clopidogrel (300 mg) was administered to all patients at least 24 hours before the intervention, and all patients were given unfractionated heparin at 100 mg/kg to achieve an activated clotting time of 250–350 s. Coronary angiography was performed according to current standard protocols via the radial or femoral approach after intracoronary injection of 100–200 μg nitroglycerin [17, 18]. At least two standardized angiographic projections for each major epicardial vessel treated with PCI were acquired, to minimize vessel overlap and foreshortening for subsequent quantitative coronary angiography (QCA) analysis.
Quantitative Coronary Angiography Analysis
An angular discrepancy of at least 30° between the projections and frame, with minimal foreshortening and vascular overlap, was chosen to obtain the optimal lesion assessment. Offline QCA was performed by an experienced investigator, who was blinded to the patients’ information, at the core laboratory of the PLA hospital with a commercially available Cardiovascular Angiography Analysis System Medis QAngio XA 7.3 (Medis medical imaging systems bv, Leiden, the Netherlands) according to the standard protocol for automated contour detection and quantification [19]. The QCA parameters of reference vessel diameter, percentage diameter stenosis ([1 - minimum lumen diameter/reference vessel diameter] × 100), and late lumen loss (difference between the postprocedure and follow-up minimum lumen diameter) were calculated. Binary restenosis was defined as >50% diameter stenosis in the target lesion or segment at angiographic follow-up. Measurements within the stented segment were defined as the in-stent analysis. The in-segment analysis included both the in-stent and 5 mm margins proximal and distal to the treated area.
Clinical and procedural data were collected by clinical research coordinators who were blinded to the patient group and study purpose. Angiographic images at baseline and during the follow-up period were saved in DICOM format for review and further QCA analyses.
Optical Coherence Tomography Image Acquisition and Analysis
OCT image acquisition was performed with a commercially available C7-XRTM OCT intravascular image acquisition system (St. Jude Medical Inc., Saint Paul, MN, USA). The OCT catheter was placed at least 10 mm distal to the target lesion of the tested artery. OCT images were analyzed in the core laboratory of the PLA hospital by an experienced investigator blinded to the patients’ information, according to the predefined study-specific standard operating procedure using the Medis QIvus Research Edition 3.0 (Medis medical imaging systems bv, Leiden, the Netherlands). The strut neointimal coverage, neointimal thickness, neointimal area, neointimal volume, mean luminal diameter, and mean luminal area were recorded as OCT parameters.
Endpoints
The primary endpoints were late catch-up in the BuMA Supreme arm compared with the XIENCE arm, defined as accumulated incidence of LLL, binary restenosis (defined as >50% diameter stenosis), thrombosis, and the extent of neointimal hyperplasia. The major secondary endpoints were the rate of imaging and clinical outcomes, including major cardiovascular events (MACEs), all-cause mortality, and the rate of stent-acquired malapposition.
Statistical Analysis
Statistical analysis was performed in SPSS version 25.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 8.0 software (San Diego, CA, USA). The data distribution was assessed with the Kolmogorov–Smirnov test. Continuous variables are presented as mean ± standard deviation for normally distributed data. Between-group differences were tested with an independent sample t test. Categorical data are presented as counts (proportions) and were compared with Pearson’s chi-squared test or Fisher’s exact test. A two-sided P value < 0.05 was considered statistically significant.
Results
Baseline Characteristics
The baseline characteristics of patients in the two arms are presented in Table 1. No significant differences were observed between device groups, except for a higher level of triglycerides in the BuMA Supreme arm (P = 0.040).
Baseline Clinical Demographics.
| BuMA Supreme, N = 24 | XIENCE, N = 20 | Overall, N = 44 | P value | |
|---|---|---|---|---|
| Male sex | 15 (62.50) | 12 (60.00) | 27 (61.36) | 0.865 |
| Age (years) | 62.54 ± 10.23 | 61.65 ± 10.49 | 62.14 ± 10.24 | 0.777 |
| Diabetes mellitus | 3 (12.50) | 6 (30.00) | 9 (20.45) | 0.261 |
| Dyslipidemia | 10 (41.67) | 3 (15.00) | 13 (29.55) | 0.054 |
| Hypertension | 15 (62.50) | 14 (70.00) | 29 (65.91) | 0.601 |
| Bleeding history | 3 (12.50) | 3 (15.00) | 6 (13.64) | 1.000 |
| BMI (kg/m2) | 24.46 ± 2.39 | 26.25 ± 3.03 | 25.28 ± 2.81 | 0.058 |
| Family history | 0 (0.00) | 1 (5.00) | 1 (2.77) | 0.722 |
| Heart failure | 0 (0.00) | 0 (0.00) | 0 (0.00) | NA |
| Atrial fibrillation | 0 (0.00) | 0 (0.00) | 0 (0.00) | NA |
| Heart rate | 72.25 ± 9.83 | 78.00 ± 11.05 | 74.86 ± 10.68 | 0.075 |
| Systolic pressure | 134.38 ± 21.19 | 129.45 ± 14.07 | 132.14 ± 18.27 | 0.380 |
| Diastolic pressure | 81.04 ± 9.81 | 79.10 ± 8.01 | 80.16 ± 8.99 | 0.482 |
| Prior MI | 0 (0.00) | 1 (5.00) | 1 (2.27) | 0.455 |
| Prior PCI | 0 (0.00) | 1 (5.00) | 1 (2.27) | 0.455 |
| Prior CABG | 0 (0.00) | 0 (0.00) | 0 (0.00) | NA |
| Arrhythmia | 1 (4.17) | 2 (10.00) | 3 (6.82) | 0.583 |
| Clinical presentation | 0.901 | |||
| Silent ischemia | 4 (16.67) | 2 (10.00) | 6 (13.64) | |
| Stable angina | 4 (16.67) | 3 (15.00) | 7 (15.91) | |
| Unstable angina | 14 (58.33) | 14 (70.00) | 28 (63.64) | |
| Unknown | 2 (8.33) | 1 (5.00) | 3 (6.82) | |
| Cerebrovascular disease | 5 (20.83) | 6 (30.00) | 11 (25.00) | 0.484 |
| Peripheral artery disease | 0 (0.00) | 1 (5.00) | 1 (2.27) | 0.201 |
| Kidney disease | 1 (4.17) | 2 (10.00) | 3 (6.28) | 0.583 |
| Procedure type | 0.493 | |||
| Emergency PCI | 22 (91.67) | 20 (100.00) | 42 (95.45) | |
| Elective PCI | 2 (8.33) | 0 (0.00) | 2 (4.55) | |
| Total cholesterol (mmol/L) | 4.26 ± 0.91 | 4.13 ± 0.73 | 4.21 ± 0.83 | 0.625 |
| Triglycerides (mmol/L) | 2.08 ± 0.94 | 1.48 ± 0.77 | 1.82 ± 0.91 | 0.040 |
| HDL-C (mmol/L) | 1.13 ± 0.33 | 1.16 ± 0.44 | 1.14 ± 0.38 | 0.783 |
| LDL-C (mmol/L) | 2.57 ± 0.71 | 2.50 ± 0.56 | 2.54 ± 0.64 | 0.731 |
Values are mean ± standard deviation, n (%).
Abbreviations: BMI, body mass index; MI, myocardial infarction, PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Primary Endpoints
The QCA assessment of the target lesion imaging data before stent implantation, in terms of reference vessel diameter, lesion length, and severity of stenosis, was comparable between groups. Stent length, stent diameter, and residual stenosis of the target lesion poststent implantation showed no significant differences between groups (Table S1). Moreover, no significant differences were observed in the rate of diameter stenosis or minimum diameter, within either the stent or the lesioned segment. Early luminal loss and LLL were equivalent between groups at the within 2 months or 2 year follow-up after stent implantation (Table S2). The diameter of stenosis in stents or segments in the BuMA Supreme and XIENCE arms between the within 2 months and 2 year follow-ups showed no significant differences (Figure 2A, B). Similar findings were observed for the minimum diameter in stents or segments in the BuMA Supreme and XIENCE stent groups (Figure 2C, D).
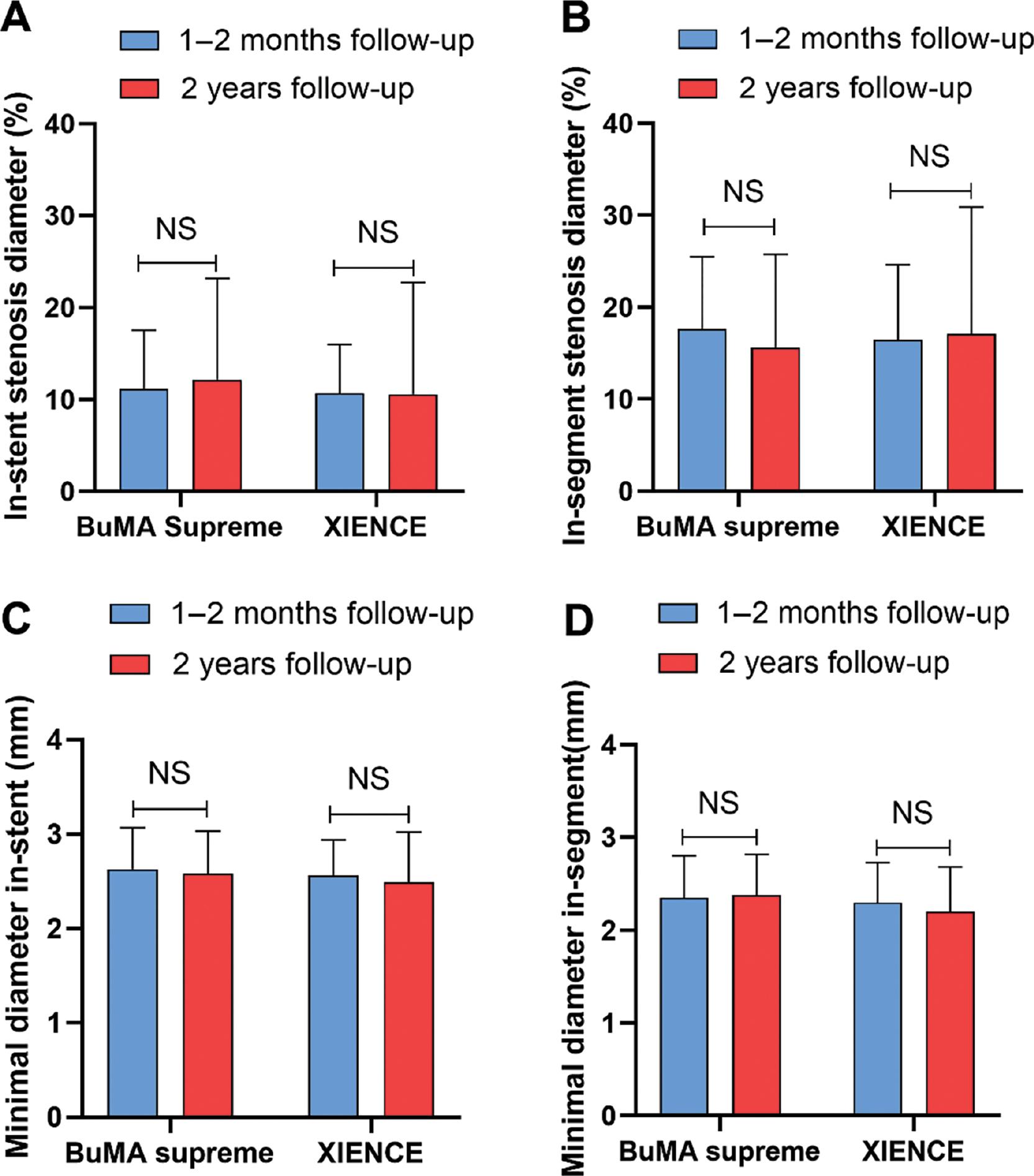
A-B: In-stent stenosis diameter or in-segment diameter in the BuMA Supreme and XIENCE arms at within 2 months and 2 year follow-ups, respectively. C-D: Minimum in-stent diameter or in-segment diameter in the BuMA Supreme and XIENCE stent groups at within 2 months and 2 year follow-ups, respectively.
BuMA group had greater strut neointimal coverage and neointimal thickness of the stent than the XIENCE group, according to the combined 1 month and 2 month OCT follow-up data analysis (P = 0.044 and P = 0.010, respectively), but these values were similar by the 2 year follow-up. A representative case of neointimal hyperplasia in the BuMA Supreme and XIENCE groups at the 2 year follow-up is shown in Figure 3. The neointimal volume was greater in the BuMA group than the XIENCE group at both the within 2 months and 2 year OCT follow-ups (P = 0.054 and P = 0.046, respectively; Table 2). Luminal loss in the BuMA Supreme group was comparable at the within 2 months (early) and 2 year (late) follow-ups, and similar results were observed in the XIENCE stent group (Figure S1). No thrombosis was detected with OCT in either group.
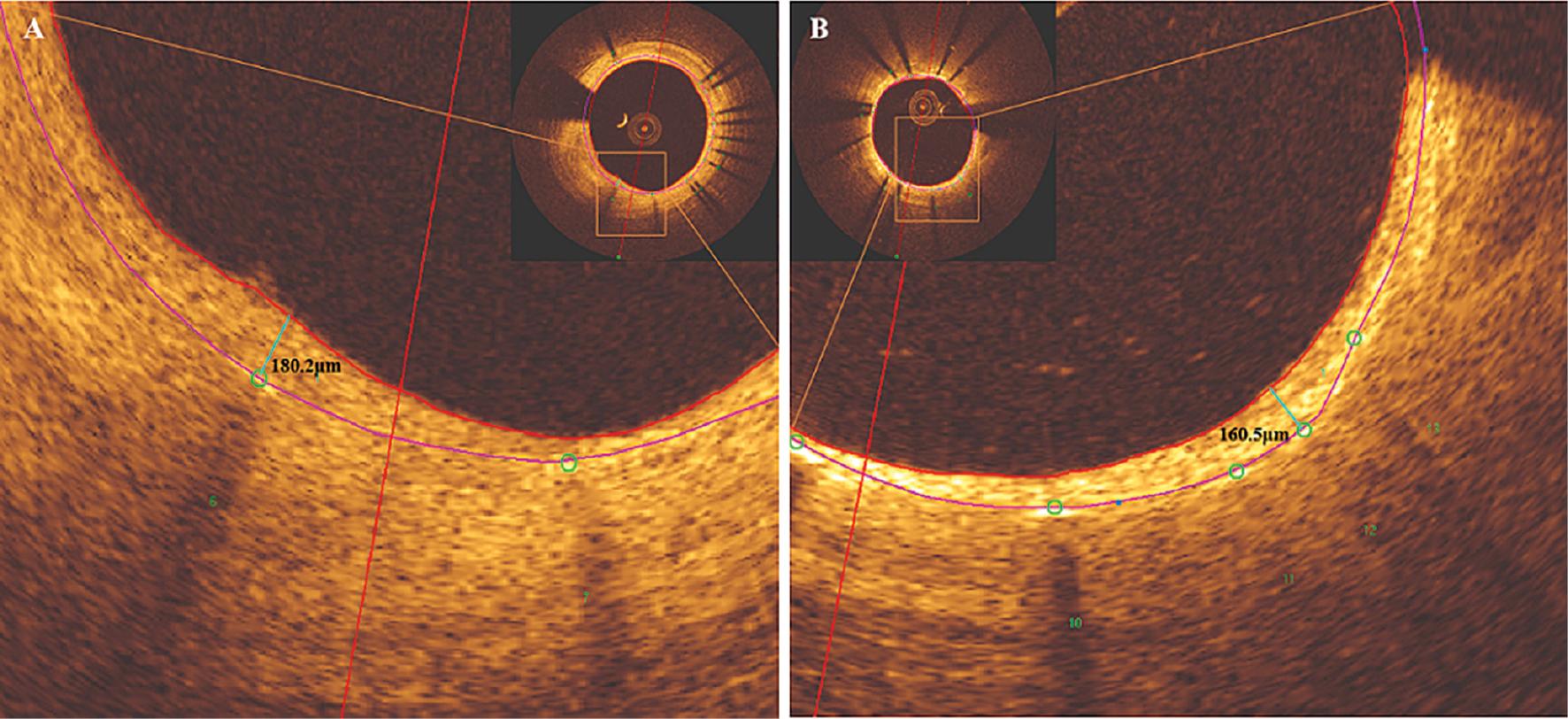
Representative Case of Neointimal Hyperplasia at the 2 Year Follow-Up. A: BuMA Supreme stent (180.2 μm) B: XIENCE stent (160.5 μm). The red arc indicates the line of neointimal hyperplasia; the purple arc indicates the line of coronary stent trabeculae; and the green circle and number indicate the scaffold.
Optical Coherence Tomography Analysis of Lesions at Follow-Up.
| BuMA Supreme, N = 24, L = 26 | XIENCE, N = 20, L = 24 | Overall, N = 44, L = 50 | P value | |
|---|---|---|---|---|
| Within 2 months follow-up | ||||
| Strut neointimal coverage (%) | 81.64 ± 16.19 | 71.26 ± 19.26 | 76.66 ± 18.31 | 0.044 |
| Neointimal thickness (μm) | 70.59 ± 38.89 | 47.35 ± 18.29 | 59.43 ± 32.66 | 0.010 |
| Neointimal area (mm2) | 0.60 ± 0.37 | 0.44 ± 0.19 | 0.52 ± 0.30 | 0.057 |
| Neointimal volume (mm3) | 17.58 ± 14.99 | 11.01 ± 6.65 | 14.43 ± 12.10 | 0.054 |
| Mean luminal diameter (mm) | 3.24 ± 0.47 | 3.20 ± 0.39 | 3.22 ± 0.43 | 0.762 |
| Mean luminal area (mm2) | 8.44 ± 2.44 | 8.21 ± 2.03 | 8.33 ± 2.23 | 0.717 |
| 2 year follow-up | ||||
| Strut neointimal coverage (%) | 99.43 ± 1.06 | 99.55 ± 0.78 | 99.49 ± 0.93 | 0.663 |
| Neointimal thickness (μm) | 163.49 ± 78.84 | 141.24 ± 71.15 | 152.81 ± 75.33 | 0.301 |
| Neointimal area (mm2) | 1.47 ± 0.75 | 1.27 ± 0.69 | 1.37 ± 0.72 | 0.343 |
| Neointimal volume (mm3) | 38.64 ± 20.48 | 28.39 ± 14.02 | 33.72 ± 18.25 | 0.046 |
| Mean luminal diameter (mm) | 2.98 ± 0.45 | 2.95 ± 0.43 | 2.97 ± 0.44 | 0.833 |
| Mean luminal area (mm2) | 7.18 ± 2.19 | 7.05 ± 2.06 | 7.11 ± 2.11 | 0.828 |
Values are mean ± standard deviation, n (%), (%).
Secondary Endpoints
No statistically significant differences in imaging and clinical endpoints, in terms of stent-acquired malapposition and MACE events, were observed between device arms at both the within 2 months and 2 year OCT follow-ups (Table S3).
Discussion
This study of second-generation biodegradable polymer sirolimus-eluting stents, comparing BuMA Supreme and XIENCE stents, showed no significant late catch-up phenomena in either device arm at the within 2 months or 2 year serial OCT follow-up. In patients enrolled in the previous PIONEER-II OCT trial with a 2 year OCT follow-up, the percentage in-stent strut neointimal coverage at within 2 months was higher in the BuMA Supreme group than the XINENCE group, similar to the results of the PIONEER-II trial [11]. The percentage diameter stenosis and minimum diameter in stents or segments did not significantly differ between the within 2 months and 2 year follow-ups, in either the BuMA Supreme or XIENCE arms. The extent of early luminal loss in the BuMA Supreme and XIENCE stent groups at within 2 months follow-up was comparable to the LLL at the 2 year follow-up, although the neointimal volume was greater in in the BuMA Supreme arm than the XIENCE arms; that is, no statistically significant increase in neointimal hyperplasia was observed.
Several pieces of evidence have indicated the existence of late catch-up in sirolimus-eluting stents compared with bare-metal stents and other drug-eluting stents (e.g., paclitaxel-eluting stents and everolimus-eluting stents) [15, 17–20]. Stent underexpansion, stent fracture, in-stent neoatherosclerosis, and stent malapposition have been reported to be associated with in-stent restenosis and thrombosis [19, 21], the main drivers of the late catch-up phenomenon. In our study, each target lesion was screened with OCT after stent implantation, thrombosis, stent underexpansion, and malapposition, and no stent fracture or neoatherosclerosis was detected at the 2 year follow-up.
The BuMA Supreme stent features a nearly equivalent thickness of the stent scaffold, whereas the XIENCE stent has a unique biodegradable polymer resulting in relatively rapid drug release. The fast strut coverage observed 1 and 2 months after BuMA Supreme stent implantation was demonstrated via OCT, on the basis of the modestly shorter drug elution, and has been suggested to decrease the risk of excessive luminal loss and in-stent thrombosis [11]. This effect persisted for at least 2 years without late catch-up phenomena and MACE events in the current OCT study. The DESSOLVE III trial [22], which studied devices in line with our current study, has indicated similar clinical outcomes at the 3 year follow-up [23]. The EVOLVE II Randomized Trial has also demonstrated that SYNERGY stents (thin-strut, bioabsorbable polymer-coated, and everolimus-eluting stents) are comparable to the durable polymer PROMUS Element Plus stent, with low rates of stent thrombosis and adverse events in 5 years of follow-up [24].
LLL has been used in many trials as a device-oriented surrogate for late catch-up. For example, in a previous study, patients treated with BuMA Supreme stents had greater in-device LLL than those treated with durable polymer zotarolimus-eluting stents at 9 months (0.29 ± 0.33 mm vs. 0.14 ± 0.37 mm, P = 0.004) [25]. LLL was comparable between the BuMA Supreme and XINENCE arms in the current 2 year follow-up (0.10 ± 0.41 mm vs. 0.16 ± 0.51 mm, P = 0.613); the compared to the above study, there was less LLL in the BuMA Supreme stents of this study. Lemos et al. have demonstrated that sirolimus-eluting stent implantation follows a presentation of all-or-none LLL [26]. A meta-analysis has identified that the optimal cutoff value for LLL in predicting TLR with reasonable sensitivity and specificity is 0.50 mm, and that an angiographic LLL > 0.50 mm is predictive of TLR incidence [27]. No MACEs or significant LLL was observed in the current study, in agreement with the study by Lemos et al. and the meta-analysis. The stenosis in-stent or in-segment diameters were comparable between the within 2 months and 2 year follow-up in both groups. Hence, late catch-up phenomena were not observed in either device arm. Although the LLL, in terms of neonatal endothelial thickness, was less than 0.5 mm between groups at the 2 year follow-up, a significant increase with respect to the within 2 months data was observed, thus suggesting the need for a longer follow-up period.
In the present era of drug-eluting stents, the tradeoffs among stent thrombosis, restenosis, and bleeding present a complex challenge [7]. The participants enrolled in the current trial had HBR and coronary heart disease, and received stent implantation, which required dual antiplatelet therapy (DAPT) to prevent thrombosis. Current guidelines recommend a short course of DAPT in patients with HBR and extensive re-endothelization [28]. In the present study, both the BuMA Supreme and XINENCE stents demonstrated relatively optimal strut coverage in a short period of time, thus suggesting favorable re-endothelization. Another trial has identified that short-term DAPT might be feasible in selected patients with favorable early strut coverage, on the basis of OCT [29]. The EVOLVE Short DAPT Study and MASTER DAPT trial support the safety of abbreviated DAPT following bioabsorbable polymer-coated stent implantation in patients with HBR [30, 31]. The faster re-endothelization and similar neointimal hyperplasia observed with the BuMA Supreme stent compared with the XIECE stent have implications for the application of the BuMA Supreme stent in patients with coronary artery disease and HBR.
Limitations
This study has several limitations. First, the small sample size precluded definite conclusions from being drawn regarding the safety and efficacy of the novel drug-eluting stents. Second, the participants enrolled in this study were from two cohorts in the PIONEER-II OCT randomized controlled trial, on the basis of the principle of patient intentionality rather than randomization. This design might have contributed to bias in the study results. Nevertheless, the participants finally enrolled in the two arms were comparable in terms of demographics, comorbidities, and biochemical parameters. Additionally, the clinical benefits of the BuMA Supreme stent compared with the XIENCE stent were not tested in patients with coronary artery disease and HBR in this trial.
Conclusions
BuMA Supreme stents are new generation drug-eluting stents with thinner struts, relatively faster drug release, and biodegradable polymers. These stents enabled faster re-endothelization than XIENCE stents at within 2 months of follow-up. Similar LLL and neointimal hyperplasia were observed. Although the BuMA Supreme stent showed greater neointimal volume, no late catch-up phenomena were observed with either device by the 2 year follow-up. Together, our findings indicated that BuMA Supreme stents have potential advantages for patients with coronary artery disease at high risk of bleeding, through decreasing the DAPT duration.